Molecular Structure from Raman Spectroscopy
Synthesis and Applications of Gold Mesostructures
Spectral Analysis in Astrobiology
Structural Analysis by Enhanced Raman Scattering (SABERS)
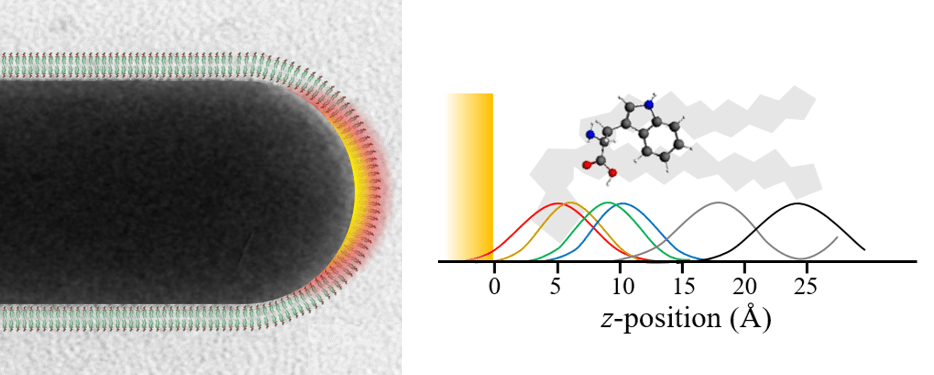
Our group’s main focus is a new method we have devleoped to solve biomembrane structures based on surface enhanced spectroscopy: SABERS (Matthews 2017). Plasmonic structures like gold nanorods focus light to a strong molecular-scale near-field at their tip. Raman scattering from molecules in this region is greatly enhanced (SERS), a phenomenon that has been widely pursued as a sensing technology. The relative strength of the different vibrational SERS peaks should depend on the orientation and position of the molecule relative to the gold surface, but SERS is difficult to interpret. We have developed ratiometric methods to remove experimental unknowns and use TDDFT to calculate the Raman tensors of complex molecules. We can then use SERS spectra to figure out how molecules sit near the gold nanorods, including molecules in a lipid bilayer. Our initial publication solved the structure of the CTAB layer on gold nanorods, the lipid bilayer structure, and the position and orientation of tryptophan (Matthews 2017). We have recently submitted a manuscript on the orientation of a voltage sensitive dye in the membrane (Hughes 2019).
#SABERS
Want to collaborate? If you are making SERS measurements from nanoparticles in solution, we could help you interpret your spectra to get structure by SABERS! Or, if you study a molecule in lipid bilayers, we don’t need much! Contact me at hafner@rice.edu.
Lipid Bilayers on Gold Nanorods
For nanoparticle sensing, therapeutics, and basic research, there is interest in combining phospholipid bilayers with the plasmonic properties of gold nanoparticles. Coincidentally, the trimenthylammonium headgroup of the surfactant that stabilizes gold nanorods (CTAB) is very similar to the terminal choline of the most common lipid: phosphatidylcholine. Nanorods can therefore be supported by lipids by simply suspending nanorods in a solution of phosphatidylcholine vesicles. We used surface enhanced Raman scattering (SERS) to study these bilayers and asses their use as models of natural biomembranes (Matthews 2015). With SERS we can observe the symmetric stretch of the choline headgroup, the bromide ion that remains on the gold surface, the double bond in the acyl chain (for DOPC), and many standard modes associated with the mehtylenes on the alkane chains. From these we found that the lipids are only electrostatically bound to the nanorod surface and rapidly exchange with lipids in solution. To confirm their natural state, we observed the acyl chain phase transition at the appropiate temperature. We also found that the lipid bilayer appears to collapse on the surface if vesicles are removed from solution, a behavior we also observed for surfactants (Lee 2011).
Past Research:
Gold Nanobelts
Long gold wires with nanoparticle-sized cross sections exhibit tunable plasmon resonances like nanorods and other shapes. We described these tunable resonances based on dark field microscopectroscopy (Anderson 2011). We also described interesting structures such as tapered and branched nanobelts (Payne 2013), as well as how the synthesis depends on the complex behavior of wormlike micelles (Payne 2014). Gold nanobelts also serve as plasmonic waveguides that provide strong quantum confinement of light due to their small size and cross sectional geometry (Anderson 2013).
Membrane Electrostatics
The atomic force microscope is highly sensitive to forces and can operate with the tip in solution. We used the AFM to map lateral charge distributions on biological samples in dilute electrolyte, including lipid membranes and DNA (Johnson 2003). An unexpected repulsive interaction between the negatively charged probe tips and zwitterionic lipids was found, which we showed was due to the lipid membrane dipole potential (Yang 2007, Yang2008). The dipole potential is a large internal potential different at the membrane interfacial region, which likely influences biomembrane function but is not widely studied because it is difficult to measure.
Plasmonic Biosensors
Plasmon resonances in gold nanoparticles are sensitive to the refractive index of their immediate nanoscale environment. The binding of molecules t the nanoparticle surface can therefore be detecting by simple spectral extinction measurements of the nanoparticles. In this way plasmonic nanoparticles can be used as low-tech biosensors. We demonstrated a real-time immunoassay based on this phenomenon that yielded the proper binding kinetics (Mayer 2008), and studied the sensitivity dependence on nanoparticle shape (Lee 2009). We also carried out the immunoassay with single nanoparticles to reach single molecule detection (Mayer 2010).
Single Particle Plasmonics
Most early measurements of nanoparticle plasmon resonances were from ensembles of similar nanoparticles suspended in colloidal solution. This left it unclear how the plasmon linewidth was affected by size differences among the nanoparticles and fundamental lifetimes of electronic excitations. We set up a dark field microspectrometer to measured the linewidth of the scattering from single gold nanoshells from Naomi Halas’s group to shed some light on the matter (Nehl 2004). We also described the plasmonics of complex particles like gold nanostars (Nehl2006).